Search
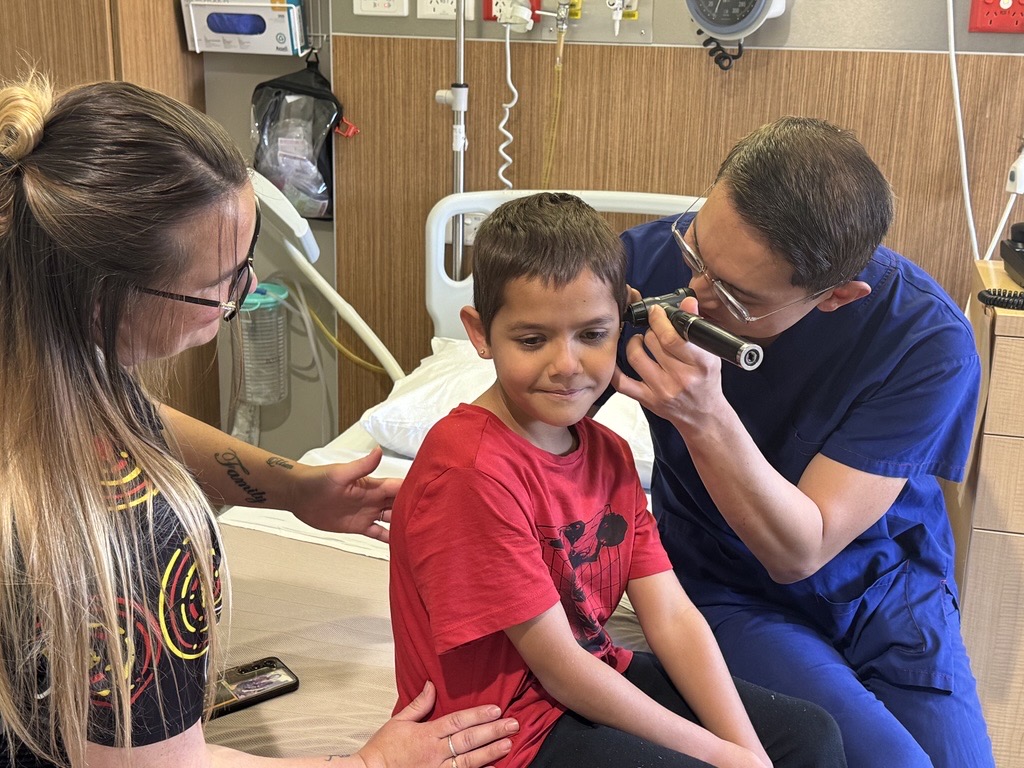
Painful ear infections and muffled sounds are a thing of the past for 100 Aboriginal children who have received free grommet surgery thanks to the Djaalinj Waakinj (listening and hearing) Ear Health program.

Researchers from the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, are partnering with Down Syndrome WA to learn more about how respiratory syncytial virus, or RSV, affects children with increased medical vulnerability.

An Australian-first study demonstrating the effectiveness of a new immunisation against respiratory syncytial virus (RSV) for babies found it to be almost 90 per cent effective in reducing hospitalisation rates and helped more than 500 WA families avoid a hospital stay.

Six researchers from The Kids Research Institute Australia have been awarded $8.9 million in prestigious Investigator Grants from the National Health and Medical Research Council.

Projects to improve outcomes for leukaemia patients and reduce skin cancer rates in young Aboriginal people have received funding through Cancer Council WA.

More than two decades of research, modelling and collaboration to develop safe and effective RSV immunisations has led to a major Federal Government roll-out of a respiratory syncytial virus (RSV) immunisation program for all pregnant women and newborn babies in 2025.
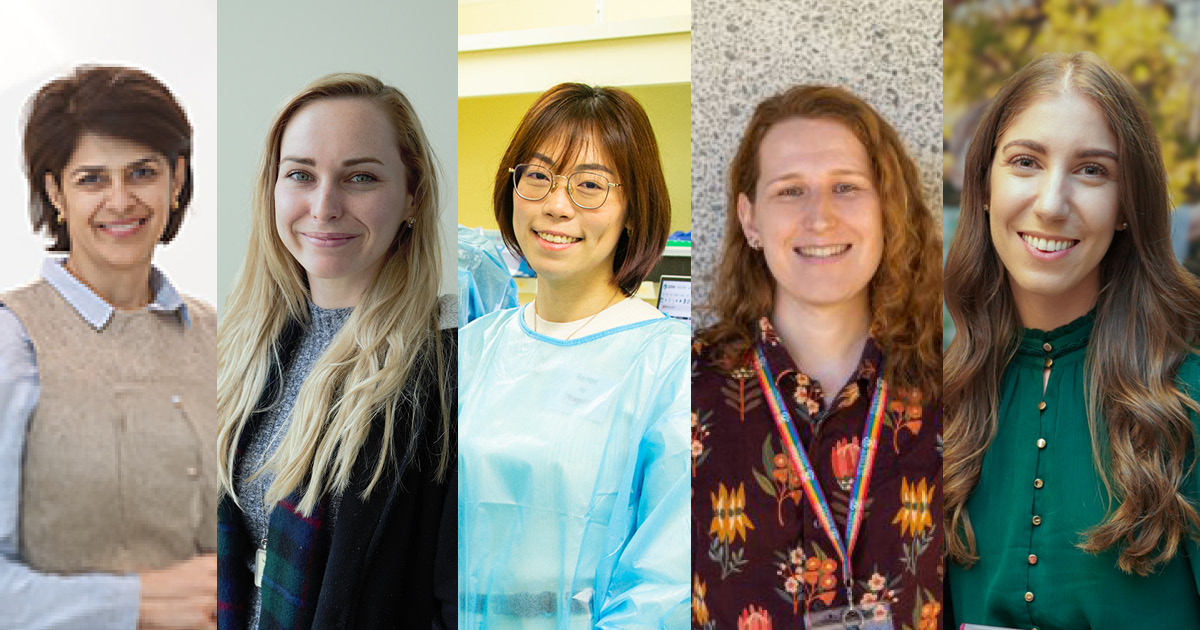
Five researchers from The Kids Research Institute Australia have been awarded three-year fellowships with the aim of keeping more WA-based PhD graduates involved in child health research.

Led by The Kids Research Institute Australia and Aboriginal health organisations in close partnership with nine Aboriginal communities in Western Australia’s Kimberley region, the five-year SToP Trial set out to identify the best possible methods to See, Treat and Prevent painful skin sores and scabies.

MEDIA ENQUIRIES Discover. Prevent. Cure. Mailing list Media contacts About The Kids Be Inspired Please direct general enquiries to our reception on (

Global circulation of respiratory syncytial virus (RSV) is shaped by human air travel with travellers hosting new strains fuelling transmission across borders, an international The Kids Research Institute Australia study found.
