Search

The FluBub Study will investigate whether giving the flu vaccine much earlier than the six months currently recommended by the National Immunisation Program will protect babies at the greatest risk of a severe influenza infection when they are most vulnerable.
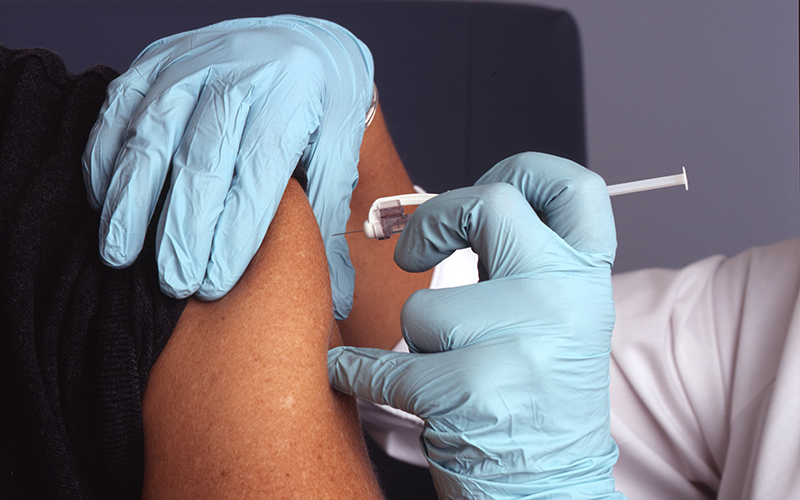
A world-leading international trial examining the immune boosting benefits of the tuberculosis vaccine, BCG, has found it does not protect healthcare workers against COVID-19.

One out of every 10 children with a bloodstream infection are infected with a multi-drug resistant organism in the nation’s first-ever surveillance study investigating the prevalence of paediatric antimicrobial resistance (AMR).

Researchers from the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, have launched an online guidance tool designed to help families and health-care providers in WA learn the best way to protect babies and young children against life-threatening respiratory syncytial virus (RSV).

Two Perth clinician-scientists have been recognised as national leaders in infectious disease research after being elected as Fellows of the esteemed Australian Academy of Health and Medical Sciences.
Gram-negative bloodstream infections are associated with significant morbidity and mortality in children. Increasing antimicrobial resistance (AMR) is reported globally, yet efforts to track pediatric AMR at a national level over time are lacking.
Primary aim was to review severe acute respiratory infections (SARI) hospitalisations caused by respiratory syncytial virus (RSV) in children aged < 2 years in paediatric hospitals in Australia. Secondary aims included RSV subtyping, assessing RSV seasonality and contributing to the World Health Organisation's RSV surveillance programme.
Annual estimates of seasonal influenza vaccine effectiveness can guide global risk communication and vaccination strategies to mitigate influenza-associated illness. We aimed to evaluate vaccine effectiveness in countries using the 2023 southern hemisphere influenza vaccine formulation.
Invasive fungal disease (IFD) occurs less frequently during treatment for solid compared to hematological malignancies in children, and risk groups are poorly defined. Retrospective national multicenter cohort data (2004-2013) were analyzed to document prevalence, clinical characteristics, and microbiology of IFD.
Antimicrobial stewardship (AMS) is crucial for optimizing antimicrobial use and restraining emergence of antimicrobial resistance. The overall increase in reported antibiotic allergies in children can pose a significant barrier to AMS, but its impact on clinical AMS care in children has not been addressed.
